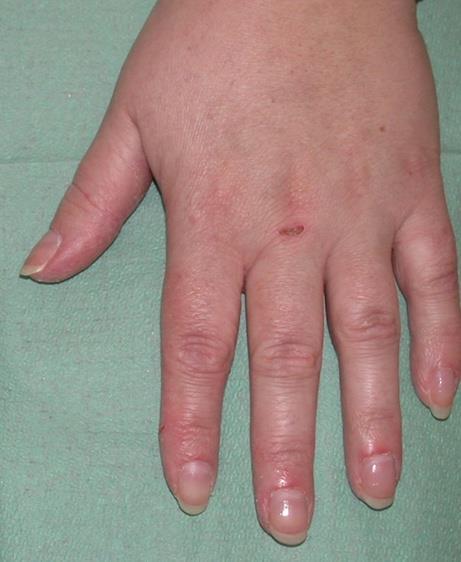
Side effects of the nails, paronychia, associated with multikinase treatment
Multikinase-inhibitors that exhibit EGFR-inhibiting properties such as vandetanib may cause nail changes, particularly paronychia, which may be complicated by pyogenic granuloma formation. 1,2
Definition: Paronychia an inflammation in the tissues adjacent to a nail on a finger or toe (nail fold), with an often tender inflammation of the nail fold.1,2 Secondary superinfection with Staphylococcus aureus may occur and may be responsible for symptomatic impairment and resistance to treatment.2 Paronychia can be extremely painful and mimics an ingrown toenail in severe cases where pyogenic granuloma of the nail fold develops.1-3
Incidence: Nail abnormalities and disorders are reported to occur with some multikinase inhibitors; they occur in up to 9% of patients treated with vandetanib whereas they are less common (≥1/100 to <1/10) with sunitinib and rare (≥1/1,000 to <1/100) with regorafenib.4-9
Grading and lesion characteristics: According to CTCAEv5.0,10 paronychia is defined as “A disorder characterised by an infectious process involving the soft tissues around the nail.” In fact, it is an inflammatory and not necessarily infectious process, although superinfection often occurs (see above). Pyogenic granuloma is not mentioned in this grading, so for this adverse event the general grading, “Skin and subcutaneous tissue disorders - Other, specify” should be used and “pyogenic granuloma” added. Within the MESTT both paronychia and pyogenic granuloma are included in the general term “nail fold changes”.11
Table 11: Grading of Paronychia according to the CTCAEv5.0
Grade |
Description |
|---|---|
1 |
Nail fold edema or erythema; disruption of the cuticle |
2 |
Local intervention indicated; oral intervention indicated (e.g., antibiotic, antifungal, antiviral); nail fold edema or erythema with pain; associated with discharge or nail plate separation; limiting instrumental ADL |
3 |
Operative intervention indicated; IV antibiotics indicated; limiting self-care ADL |
ADL: Activities of Daily Living
Table 12: Grading of Pyogenic Granuloma according to CTCAEv5.0; Skin and subcutaneous tissue disorders - Other, specify
Grade |
Description |
|---|---|
1 |
Asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated |
2 |
Moderate; minimal, local or non-invasive intervention indicated; limiting age appropriate instrumental ADL |
3 |
Severe or medically significant but not immediately life-threatening; hospitalisation or prolongation of existing hospitalisation indicated; disabling; limiting self-care ADL |
4 |
Life-threatening consequences; urgent intervention indicated |
5 |
Death |
ADL: Activities of Daily Living
Table 13: Grading of Nail fold changes according to MESTT11
Grade |
Description |
|---|---|
1 |
Disruption or absence of cuticle; OR erythema |
2 |
Erythematous/tender/painful; OR pyogenic granuloma; OR crusted lesions: OR any fold lesion interfering on instrumental ADL |
3 |
Periungual abscess; OR fold changes interfering with self-care ADL |
ADL: Activities of Daily Living
Pathological features: paronychia is probably caused by horny material, caused by cell growth arrest and differentiation, that gets stuck between the nail plate and nail fold and pricks like a needle into the nail fold.1 These changes in epithelial homeostasis cause the disruption of the cutaneous barrier leading to infection.
Onset: Paronychia typically occurs within 4-8 weeks of therapy with vandetanib.
Resolution: Nail changes are reported to resolve spontaneously.12 See also Prophylaxis and treatment - reactive management – nail changes, paronychia.
Grade 1 Paronychia
Nail fold oedema or erythema; disruption of the cuticle
Grade 2 Paronychia
Local intervention indicated; oral intervention indicated (e.g., antibiotic, antifungal, antiviral); nail fold oedema or erythema with pain; associated with discharge or nail plate separation; limiting instrumental ADL.
Grade 3 Paronychia
This photograph shows a severe grade 3 paronychia with pyogenic granuloma. Surgical intervention or IV antibiotics are indicated; limiting self-care ADL.
Related Links
- Common Terminology Criteria for Adverse Events (CTCAE)
- MASCC EGFR Inhibitor Skin Toxicity Tool (MESTT)
References
- Segaert S, et al. Eur J Cancer. 2009;45 Suppl 1:295-308.
- Robert C, et al. Lancet Oncol. 2005;6:491-500.
- Balagula Y, Lacouture ME, Cotliar JA. J Support Oncol. 2010;8:149-161.
- European Medicines Agency. Caprelsa (vandetanib) Summary of Product Characteristics 2019.
- Food and Drug Administration. Caprelsa (vandetanib) Prescribing Information 2018.
- European Medicines Agency. Sutent (sunitinib) Summary of Product Characteristics 2019.
- Food and Drug Administration. Sutent (sunitinib) Prescribing Information 2019.
- European Medicines Agency. Stivarga (regorafenib) Summary of Product Characteristics 2018.
- Food and Drug Administration. Stivarga (regorafenib) Prescribing Information 2019.
- National Cancer Institute Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events and Common Toxicity Criteria [v5.0]. 27 November 2017. (Accessed 15 April 2019).
- MASCC EGFR Inhibitor Skin Toxicity Tool (MESTT). (Accessed 15 April 2019).
- McLellan B, Kerr H. Dermatol Ther. 2011;24:396-400.