Keratoacanthoma and squamous cell carcinoma associated with multikinase inhibitor treatment
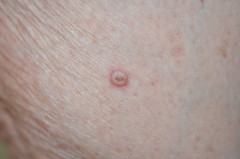
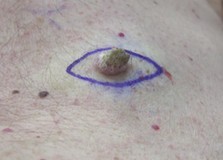
Definition: The development of cutaneous premalignant and malignant lesions is rarely derived from multikinase inhibitor treatment (but can occur especially with sorafenib).
Incidence: Squamous cell carcinoma/keratoacanthoma occurs rarely (up to 1 in 1,000) and commonly (up to 1 in 10) in patients treated with regorafenib and sorafenib, respectively.1, 2
Onset: In patients with renal cell carcinoma treated with sorafenib or sunitinib, the median treatment duration before the diagnosis of cutaneous malignancies, including squamous cell carcinoma and basal cell carcinoma, was 13.5 months.3 These lesions are caused by paradoxical activation of mitotic cell pathways by the multikinase inhibitor onto a genetic background of activating mutations of the same pathway caused by sun exposure.4
Resolution: Resolution of cutaneous malignancies with sorafenib was seen following discontinuation of therapy, with no new or persisting keratoacanthoma or squamous cell carcinomas observed.5, 6 See also Prophylaxis and treatment - reactive management – keratoacanthoma and squamous cell carcinoma.
Keratoacanthomas
Squamous cell carcinoma
References
- European Medicines Agency. Stivarga (regorafenib) Summary of Product Characteristics 2018.
- European Medicines Agency. Nexavar (sorafenib) Summary of Product Characteristics 2018.
- Breaker K, Naam M, La Rosa FG. Dermatol Surg. 2013;39:981–987.
- Oberholzer PA, et al. J Clin Oncol. 2012;30: 316-321.
- McLellan B, Kerr H. Dermatol Ther. 2011;24:396–400.
- Arnault JP, et al. J Clin Oncol. 2009;27:e59–e61.