Side effects of the hair, associated with multikinase treatment
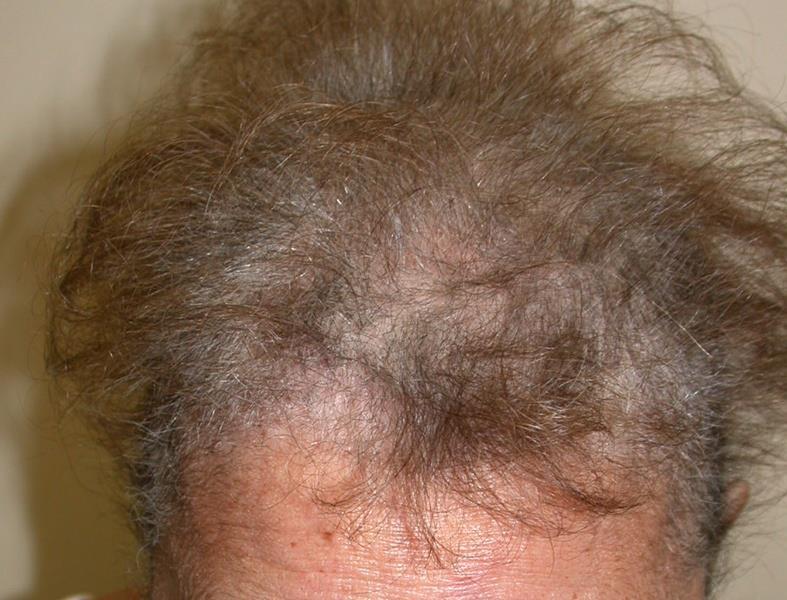
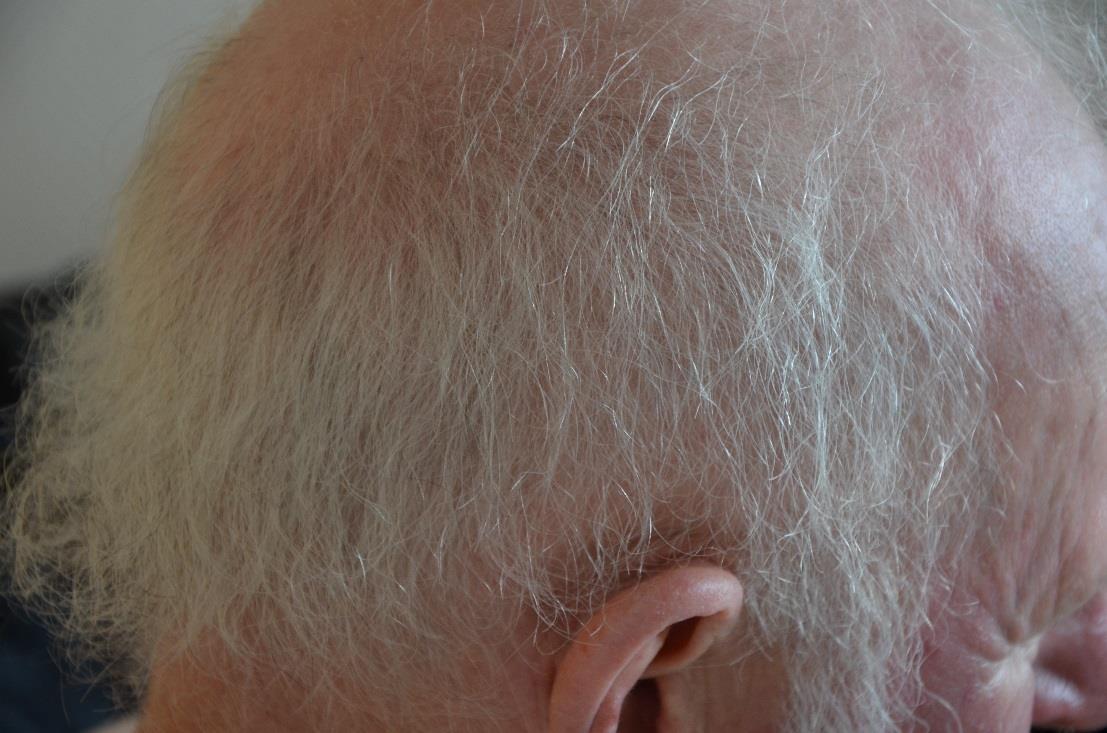
Definition: The main hair change occurring with multikinase inhibitors is alopecia, defined as a decrease in hair density compared to normal.1 Hair colour changes can also occur, for example greying of hair which has been reported with imatinib and sunitinib.2 Repigmentation of grey hair has also been reported with imatinib.3 Hair changes may include elongation and curling of the eyelashes (trichomegaly) and hypertrichosis (including facial hirsutism) or a decrease in hair growth kinetics, whereas scalp and extremity hair may become curly, fine and brittle.2,4
Incidence: Alopecia is reported as a common (≥1/100 to <1/10) or very common (≥1/10) adverse reaction occurring with multikinase inhibitors including, cabozantinib, lenvatinib, regorafenib, dasatinib, pazopanib and sorafenib. 5-19 Hair colour changes (greying of hair) are also common or very common with cabozantinib, pazopanib, vandetanib, sunitinib and imatinib5-8, 16-17, 20-25 Repigmentation of grey hair has been reported in a small proportion of patients treated with imatinib.3 It has been shown that in general patients treated with targeted therapies have an increased risk of all-grade alopecia compared to placebo, but a lower risk when compared to chemotherapy.26
Grading and lesion characteristics: According to the CTCAEv.5.0,1 alopecia is defined as “A disorder characterised by a decrease in density of hair, compared to normal, for a given individual at a given age and body location.” With targeted therapy, thinning hair is reported regularly, while partial alopecia is reported less frequently.
Table 14: Grading of Alopecia according to the CTCAEv5.0
Grade |
Description |
|---|---|
1 |
Hair loss of <50% of normal for that individual, that is not obvious from a distance but only on close inspection; a different hair style may be required to cover the hair loss, but it does not require a wig or hair piece to camouflage |
2 |
Hair loss of ≥50% normal for that individual, that is readily apparent to others; a wig or hair piece is necessary if the patient desires to completely camouflage the hair loss; associated with psychosocial impact |
Table 15: Grading of Scalp hair loss or Alopecia according to MESTT27
Grade |
Description |
|---|---|
1 |
Terminal hair loss < 50% of normal for that individual, that may or may not be noticeable to others but is associated with increased shedding and overall feeling of less volume. May require different hair style to cover but does not require hairpiece to camouflage |
2 |
2A Hair loss associated with marked increase in shedding and 50%-74% loss compared to normal for that individual. Hair loss is apparent to others, may be difficult to camouflage with change in hair style and may require hairpiece 2B Marked loss of at least 75% hair compared to normal for that individual with inability to camouflage except with a full wig OR new cicatricial hair loss documented by biopsy that covers at least 5% scalp surface area. May impact on functioning in social, personal or professional situations |
Onset: Alopecia with sorafenib therapy is reported to occur around week 3- 15 of treatment initiation and hair may regrow even while patients are still on treatment during weeks 3–15 of therapy; 28 hair colour changes (depigmentation) can be seen 5-6 weeks after treatment initiation and is reversible 2-3 weeks after treatment is completed.29
Resolution: Alopecia is temporary and may spontaneously resolve in some patients despite continued multikinase inhibitor therapy.2 Also see Prophylaxis and treatment - reactive management - hair changes page.
Grade 1 Alopecia
Hair loss of <50% of normal for that individual that is not obvious from a distance but only on close inspection; a different hair style may be required to cover the hair loss, but it does not require a wig or hair piece to camouflage.
Grade 2 Alopecia
Hair loss of ≥50% normal for that individual that is readily apparent to others; a wig or hair piece is necessary if the patient desires to completely camouflage the hair loss; associated with psychosocial impact.
Related Links
- Common Terminology Criteria for Adverse Events (CTCAE)
- MASCC EGFR Inhibitor Skin Toxicity Tool (MESTT)
References
- National Cancer Institute Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events and Common Toxicity Criteria [v5.0]. 27 November 2017. (Accessed 15 April 2019).
- McLellan B, Kerr H. Dermatol Ther. 2011;24:396–400.
- Etienne G. N Engl J Med. 2002; 347:446.
- Segaert S, et al. Eur J Cancer. 2009;45 Suppl 1:295-308.
- European Medicines Agency. Cabometyx (cabozantinib) Summary of Product Characteristics 2019.
- Food and Drug Administration. Cabometyx (cabozantinib) Prescribing Information 2019.
- European Medicines Agency. Cometriq (cabozantinib) Summary of Product Characteristics 2019.
- Food and Drug Administration. Cometriq (cabozantinib) Prescribing Information 2018.
- European Medicines Agency. Kisplyx (lenvatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Lenvima (lenvatinib) Summary of Product Characteristics 2019.
- Food and Drug Administration. Lenvima (lenvatinib) Prescribing Information 2018.
- European Medicines Agency. Stivarga (regorafenib) Summary of Product Characteristics 2018.
- Food and Drug Administration. Stivarga (regorafenib) Prescribing Information 2019.
- European Medicines Agency. Sprycel (dasatinib) Summary of Product Characteristics 2019.
- Food and Drug Administration. Sprycel (dasatinib) Prescribing Information 2018.
- European Medicines Agency. Votrient (pazopanib) Summary of Product Characteristics 2018.
- Food and Drug Administration. Votrient (pazopanib) Prescribing Information 2017.
- European Medicines Agency. Nexavar (sorafenib) Summary of Product Characteristics 2018.
- Food and Drug Administration. Nexavar (sorafenib) Prescribing Information 2018.
- European Medicines Agency. Caprelsa (vandetanib) Summary of Product Characteristics 2019.
- Food and Drug Administration. Caprelsa (vandetanib) Prescribing Information 2018.
- European Medicines Agency. Sutent (sunitinib) Summary of Product Characteristics 2019.
- Food and Drug Administration. Sutent (sunitinib) Prescribing Information 2019.
- European Medicines Agency. Glivec (imatinib) Summary of Product Characteristics 2019.
- Food and Drug Administration. Gleevec (imatinib) Prescribing Information 2018.
- Belum et al. Ann Oncol. 2015;26:2496-502.
- MASCC EGFR Inhibitor Skin Toxicity Tool (MESTT). (Accessed 15 April 2019).
- Autier J, et al. Arch Dermatol. 2008;144:886–892.
- Robert C, et al. J Am Acad Dermatol. 2009;60:299–305.