Predictive and/or Prognostic Biomarkers of (Potential) Clinical Relevance
PD-L1 Staining
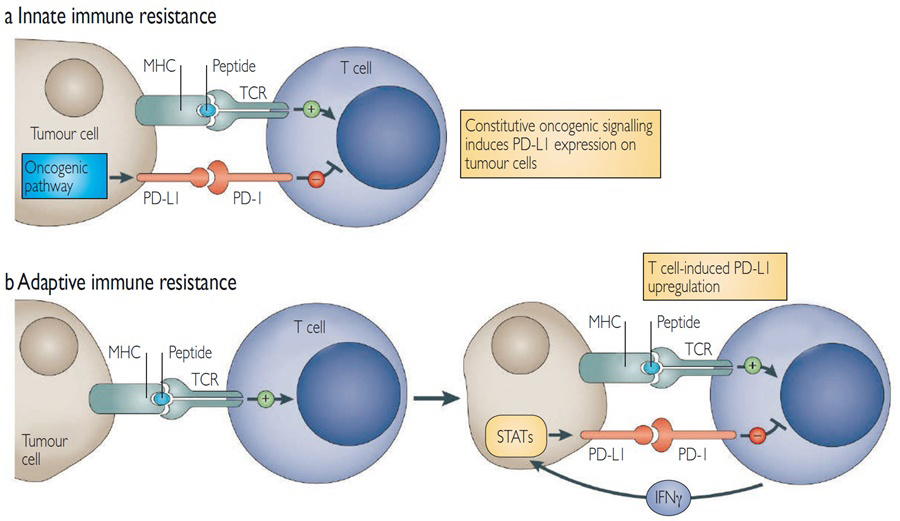
The tumoural expression of PD-L1, assessed by immunohistochemistry (IHC) staining, has been identified as a biomarker associated with a higher chance of tumour response in patients treated with anti-PD-L1 antibodies and a better OS in multiple tumour types. The PD-L1 status of a tumour relies both on the IHC staining kit and the scoring methods. Because of the heterogeneity of assays, there is no consensus on a cut-off defining a PD-L1-high tumour. PD-L1 can be expressed either constitutively via an oncogenic pathway or induced by inflammatory cytokines such as interferon-gamma (IFN-λ) (Figure 2). IFN-λ can also lead to the upregulation of PD-L1 at the surface of any cell in the tumour microenvironment, and activated T cells could be double-positive for PD-1 and PD-L1.
Figure 2: Expression of PD-L1 on tumour cells
Pembrolizumab is, at present, the only anti-PD-1 antibody to be approved by the Food and Drug Administration (FDA) for a selected population of PD-L1-positive patients in non-small cell lung cancer (NSCLC) and gastric cancer.
Inflammatory Tumours and CD8+ T cells
In several tumour types, tumours with IFN-λ gene expression profile and a high level of tumour-infiltrative CD8+ T cells have better responses and survival following anti-PD-(L)1 therapy.
Mutational Load
Tumours with a high mutational load have been correlated with OS benefits following treatment with ipilimumab in MM, with pembrolizumab in NSCLC and with atezolizumab in bladder cancers. It is currently believed that a high tumour mutation burden (TMB) yields numerous immunogenic cancer cell neo-epitopes, that may be recognised by T cells upon presentation by MHC molecules. However, the TMB seems to be a prognostic marker independent of the intratumoural inflammatory gene expression profile. The assessment of TMB is currently evaluated from either tumour samples or circulating tumour DNA.
Mismatch Repair Status
Tumours with DNA mismatch repair deficiency (dMMR)/microsatellite instability-high (MSI-H) have shown great sensitivity to anti-PD-(L)1 therapies. It is currently believed that tumours harbouring an erroneous MMR system will accumulate DNA mutations, which can lead to the presence of high levels of mutation-associated neoantigens, most recognised by immune cells. Tumours identified as having a dMMR/MSI-H status are eligible for treatment with pembrolizumab in the USA.
Blood Biomarkers
A high neutrophil to lymphocyte ratio has been associated with poor outcomes in patients treated with ipilimumab and anti-PD-(L)1 therapy across different tumour types. High levels of serum lactate dehydrogenase (LDH) or soluble CD25 have been associated with poor prognosis, although none is currently used in the clinic.
Microbiota
In preclinical models, the gut microbiota has been identified as a key modulator of the immune system by enhancing T cell activation and infiltration into tumours. The impact of the gut microbiome on anti-PD-(L)1 efficacy remains to be demonstrated in humans, but is currently under active investigation.