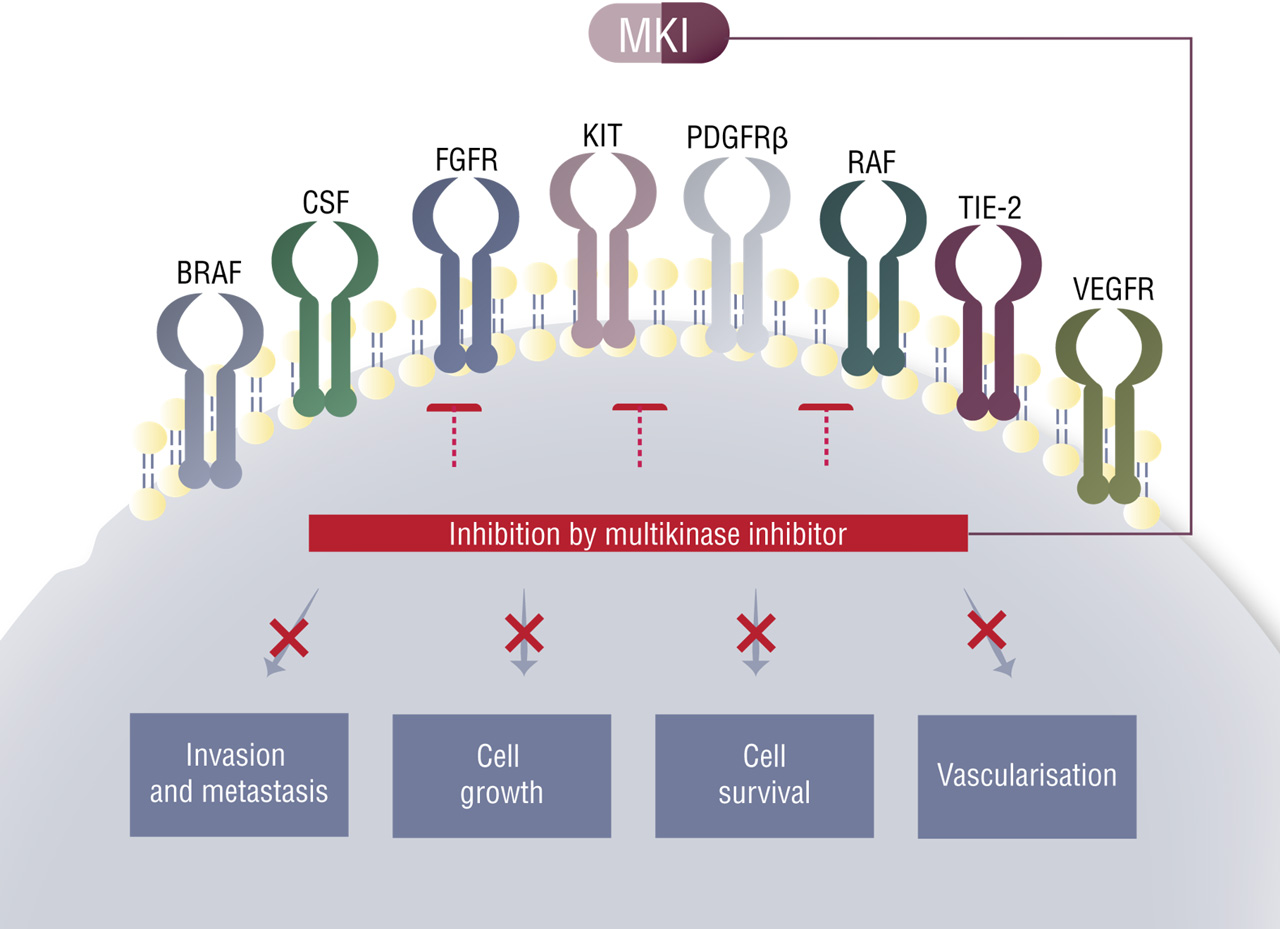
Mechanism of multikinase inhibitor action
Tumour development involves the dysregulation of normal cell processes such as cell growth and proliferation, as well as angiogenesis (the formation of new blood vessels). 1 Kinases are enzymes the control such processes and how various signal are transduced from the extracellular to the intracellular tumour environment. A range of receptor kinases are involved in these processes, including vascular endothelial growth factor receptor (VEGF), platelet-derived growth factor receptor (PDGFR), stem cell factor (c-KIT), Flt3, fibroblast growth factor receptor (FGFR), which may be found on the tumor cells, tumour vasculature or stroma and whose function is dysregulated (hyperactivated) during tumour formation and progression.1
Tumour growth may be prevented by inhibiting the action of these hyperactivated receptor kinases, and as tumour progression usually involves the action of multiple kinases rather than just one, it is logical to target multiple kinases.1 This can be achieved through the use of a combination of selective targeted agents (targeting preferentially one kinase receptor), or through a single inhibitor that simultaneously targets multiple kinases (see figure below). These latter types of inhibitors are referred to as multikinase inhibitors and, at present, include cabozantinib, imatinib, lenvatinib, regorafenib, sorafenib, sunitinib, and vandetinib. The kinase inhibition profile for each of these drugs is shown in the table below.
Figure: Mode of action of multikinase inhibitors
Table 36: Kinase inhibition profile of multikinase inhibitors
References
- Lacouture ME, et al. The Oncologist. 2008;13:1001-1011.
- European Medicines Agency. Stivarga (regorafenib) Summary of Product Characteristics 2018.
- European Medicines Agency. Nexavar (sorafenib) Summary of Product Characteristics 2018.
- European Medicines Agency. Caprelsa (vandetanib) Summary of Product Characteristics 2019.
- European Medicines Agency. Sutent (sunitinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Glivec (imatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Cabometyx (cabozantinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Cometriq (cabozantinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Kisplyx (lenvatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Lenvima (lenvatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Sprycel (dasatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Votrient (pazopanib) Summary of Product Characteristics 2018.
- European Medicines Agency. Rydapt (midostaurin) Summary of Product Characteristics 2018.
- Food and Drug Administration. Stivarga (regorafenib) Prescribing Information 2019.
- Food and Drug Administration. Nexavar (sorafenib) Prescribing Information 2018.
- Food and Drug Administration. Caprelsa (vandetanib) Prescribing Information 2018.
- Food and Drug Administration. Sutent (sunitinib) Prescribing Information 2019.
- Food and Drug Administration. Gleevec (imatinib) Prescribing Information 2018.
- Food and Drug Administration. Cabometyx (cabozantinib) Prescribing Information 2019.
- Food and Drug Administration. Cometriq (cabozantinib) Prescribing Information 2018.
- Food and Drug Administration. Lenvima (lenvatinib) Prescribing Information 2018.
- Food and Drug Administration. Sprycel (dasatinib) Prescribing Information 2018.
- Food and Drug Administration. Votrient (pazopanib) Prescribing Information 2017.
- Food and Drug Administration. Rydapt (midostaurin) Prescribing Information 2018.