HFSR linked to multikinase inhibitor treatment
Definition: Hand-foot skin reaction (HFSR) refers to symptoms affecting the hands and/or feet associated with multikinase inhibitors.1
Hand-foot skin reaction is distinct from the hand-foot syndrome (HFS). HFS, also known as palmar-plantar erythrodysesthesia, is associated with chemotherapy agents such as 5-fluorouracil and capecitabine.1 HFS presents with diffuse painful oedema and redness of palms and soles. Whereas HFSR is dose-dependent and characteristically localizes to areas of pressure or friction on the skin, such as on the heels, metatarsal heads, and areas of friction caused by shoes or manual labour. Lesions are sharply demarcated, erythematous, oedematous, painful and very tender blisters that evolve into inflamed and painful calluses.2,3
Incidence: Apart from imatinib, dasatinib, pazopanib and midostaurin, in which the frequency cannot be estimated on currently available data, HFSR is reported with an incidence of between ≥1% to approximately 50% of all patients treated with multikinase inhibitors, depending on the particular drug and tumour type.4-15
Grading and lesion characteristics: The CTCAEv5.0, 16 has no specific grading for HFSR. However, the grading used for HFS (palmar-plantar erythrodysesthesia) can also be used for HFSR. It defines HFS as “A disorder characterised by redness, marked discomfort, swelling, and tingling in the palms of the hands or the soles of the feet.”
Table 5: Grading of Palmar-Plantar Erythrodysesthesia* Syndrome According to the CTCAEv5.0
Grade |
Description |
|---|---|
1 |
Minimal skin changes or dermatitis (e.g., erythema, oedema, or hyperkeratosis) without pain. |
2 |
Skin changes (e.g., peeling, blisters, bleeding, fissures, edema, or hyperkeratosis) with pain; limiting instrumental ADL. |
3 |
Severe skin changes (e.g., peeling, blisters, bleeding, fissures, edema, or hyperkeratosis) with pain; limiting self care ADL. |
ADL: Activities of Daily Living
*palmar-plantar erythrodysesthesia syndrome is clearly distinct from HFSR but as there is no grading for HFSR in CTCAE we use the classification for palmar-plantar erythrodysesthesia syndrome but bearing in mind that it is different from HFSR
Pathological features: Include epidermal keratinocyte apoptosis, dyskeratosis and vacuolar degeneration with intraepidermal blister formation followed by massive acanthosis, papillomatosis and parakeratotic hyperkeratosis.2
Onset: Initial symptoms of HFSR (often a mild-to-moderate cutaneous reaction)17,18 with multikinase inhibitors typically appear within the first 2–6 weeks of treatment and include tenderness, paresthesia, dysesthesia, intolerance to contact with hot object.19,20 Several weeks after initial symptoms, blisters followed by thickened or hyperkeratotic skin may appear on frictional or weight-bearing areas of the skin, which are often painful and impair range of motion, and function, and weight bearing.18
Resolution: Symptoms of HFSR may diminish as treatment continues and usually it can be successfully controlled with appropriate management.18-23 Also see Prophylaxis and treatment - reactive management - Hand-foot Skin Reaction page.
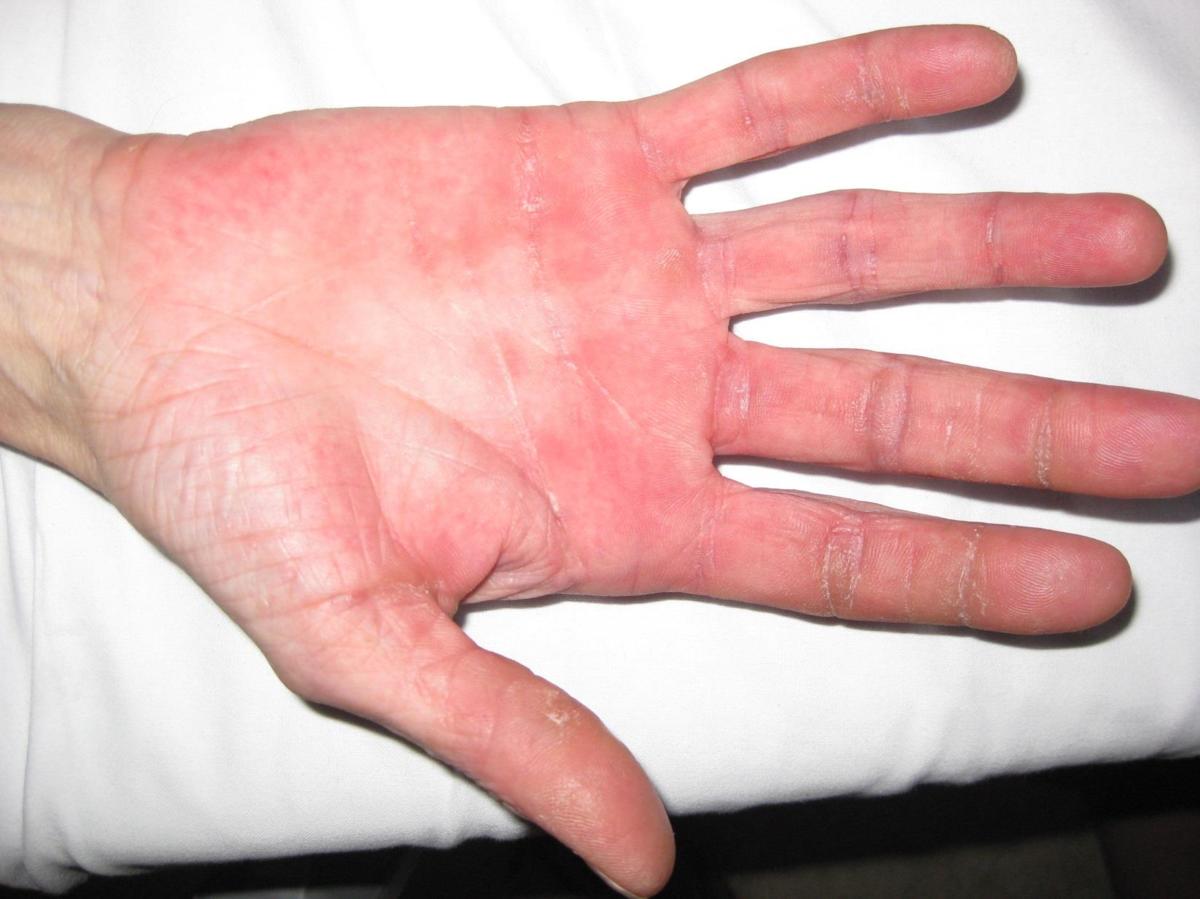
Grade 1 (mild) Hand-foot skin reaction
The NCI-CTCAE v5.0 does not grade HFSR but definition for grade 1 Palmar-plantar erythrodysesthesia syndrome: Minimal skin changes or dermatitis (e.g., erythema, oedema, or hyperkeratosis) without pain and not limiting instrumental ADL.
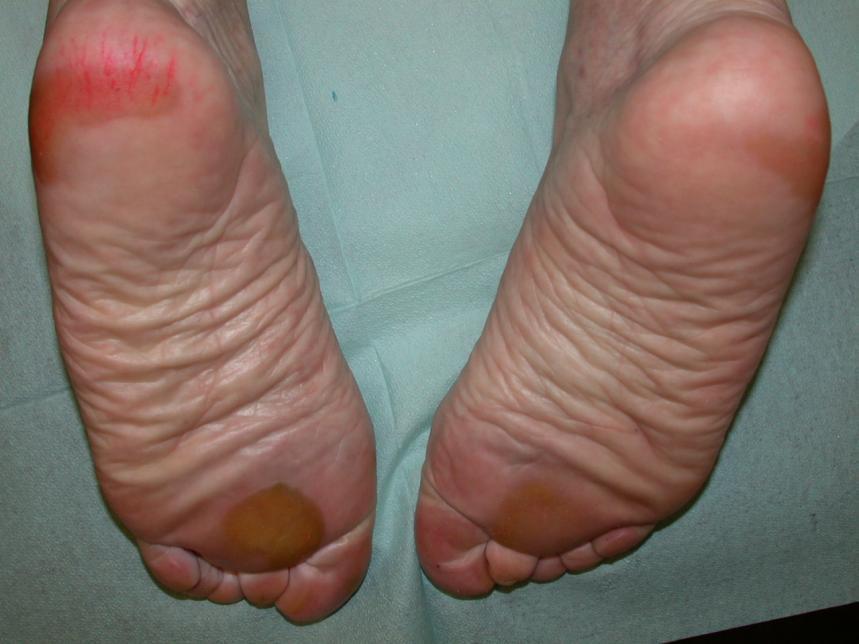
Grade 2 (moderate) Hand-foot skin reaction
The corresponding NCI-CTCAE v5.0 definition for grade 2 Palmar-plantar erythrodysesthesia syndrome states: skin changes (e.g., peeling, blisters, bleeding, oedema, or hyperkeratosis) with pain; slightly limiting instrumental ADL.
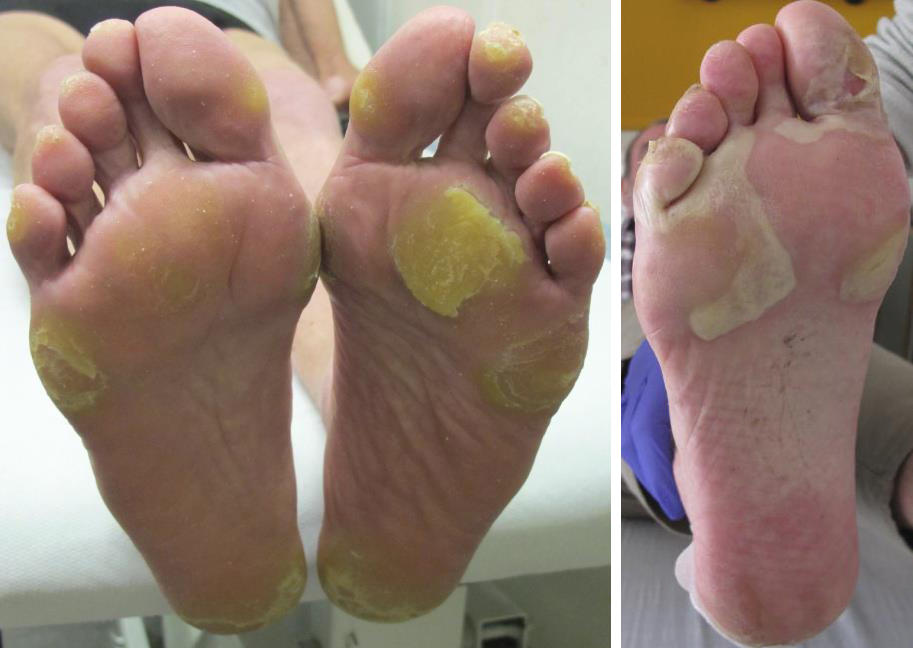
Grade 3 (Severe) Hand-foot skin reaction
The NCI-CTCAE v5.0 definition for grade 3 Palmar-plantar erythrodysesthesia syndrome states: Severe skin changes (e.g., peeling, blisters, bleeding, oedema, or hyperkeratosis) with pain; limiting self-care ADL.
Related Links
References
- Gomez P, Lacouture ME. The Oncologist. 2011;16:1508 –1519.
- Segaert S, et al. Eur J Cancer. 2009;45:295-308.
- Manchen E, et al. J Support Oncol. 2011;9:13-23
- European Medicines Agency. Stivarga (regorafenib) Summary of Product Characteristics 2018.
- European Medicines Agency. Nexavar (sorafenib) Summary of Product Characteristics 2018.
- European Medicines Agency. Caprelsa (vandetanib) Summary of Product Characteristics 2019.
- European Medicines Agency. Sutent (sunitinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Glivec (imatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Cabometyx (cabozantinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Cometriq (cabozantinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Kisplyx (lenvatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Lenvima (lenvatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Sprycel (dasatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Votrient (pazopanib) Summary of Product Characteristics 2018.
- European Medicines Agency. Rydapt (midostaurin) Summary of Product Characteristics 2018.
- National Cancer Institute Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events and Common Toxicity Criteria [v5.0]. 27 November 2017. (Accessed 15 April 2019).
- Wood LS, et al. Commun Oncol. 2010;7:23–29.
- Lacouture ME, et al. Oncologist. 2008;13:1001–1011.
- De Wit M, et al. Support Care Cancer. 2014;22:837–846.
- McLellan B, Kerr H. Dermatol Ther. 2011;24:396–400.
- Autier J, et al. Arch Dermatol. 2008;144:886–892.
- Anderson R, et al. Oncologist. 2009;14:291–302.
- McLellan B, Ann Oncl. 2015; 10:2017-2026.