Maculopapular rashes linked to multikinase inhibitor treatment
Definition: maculopapular rash is also referred to as morbilliform rash, maculopapular eruption, morbilliform exanthema, and maculopapular exanthema.1 It is characterized by a flat, red area on the skin that is covered with small confluent bumps (papules), and also includes erythema. It frequently affects the upper trunk, spreading centripetally and is associated with pruritus.1
Incidence: Rash, which includes both papulopustular maculopapular rash, is reported to be a very common skin toxicity occurring in over 10% of patients receiving treatment with all the multikinase inhibitors discussed on this website.2-13
Grading and lesion characteristics: According to the CTCAEv5.0,1 maculopapular rash is defined as, “A disorder characterised by the presence of macules (flat) and papules (elevated). It is one of the most common cutaneous adverse events, frequently affecting the upper trunk, spreading centripetally and associated with pruritus.” CTCAEv5.0, grading of maculopapular rash remains difficult: grading of the affected parts of the body is related to the body surface area (BSA), the calculated surface area of the whole body.
Table 4: Grading of Maculopapular Rash According to the CTCAEv5.0
Grade |
Description |
|---|---|
1 |
Macules/papules covering <10% BSA with or without symptoms (e.g., pruritus, burning, tightness) |
2 |
Macules/papules covering 10-30% BSA with or without symptoms (e.g., pruritus, burning, tightness); limiting instrumental ADL; rash covering >30% BSA with or without mild symptoms |
3 |
Macules/papules covering >30% BSA with moderate or severe symptoms; limiting self-care ADL |
ADL: Activities of Daily Living, BSA: Body Surface Area
Onset: Rash with multikinase therapy occurs early in therapy typically within the first cycle of therapy, 14-16 and often as early as within 1–3 weeks of treatment initiation.17
Resolution: The incidence of rash may be considerably reduced in subsequent cycles of multikinase inhibitor therapy14 and is reported to disappear without treatment in less than 2 months.16 In several cases, rash is reported to resolve within days of initiating management with topical or systemic treatments.17 Also see Prophylaxis and treatment - reactive management - Maculopapular rash page.
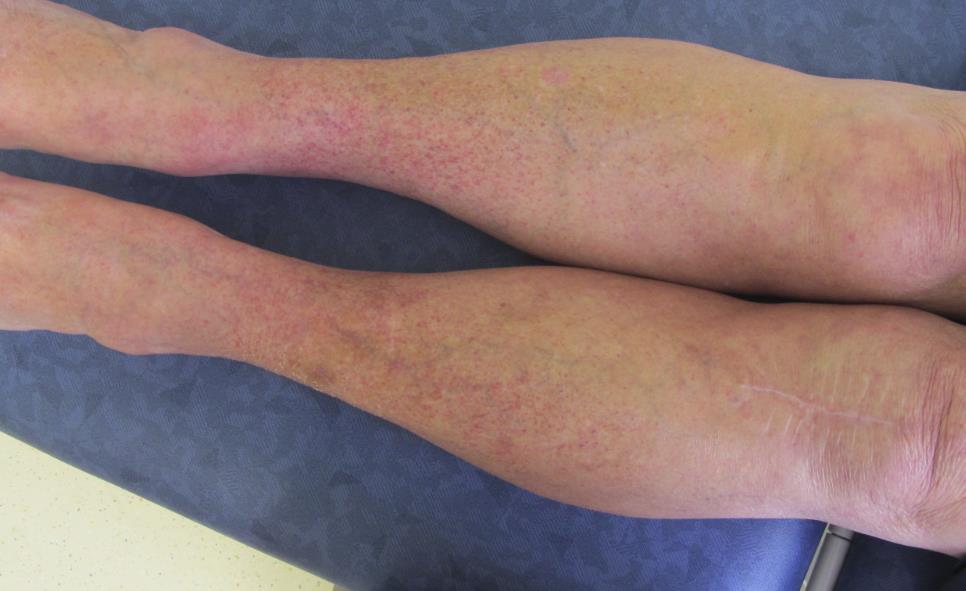
Grade 1 (mild) rash
The NCI-CTCAE v5.0 definition for ‘grade 1 Rash maculopapular’ states: Macules/papules covering <10% BSA with or without symptoms (e.g., pruritus, burning, tightness).
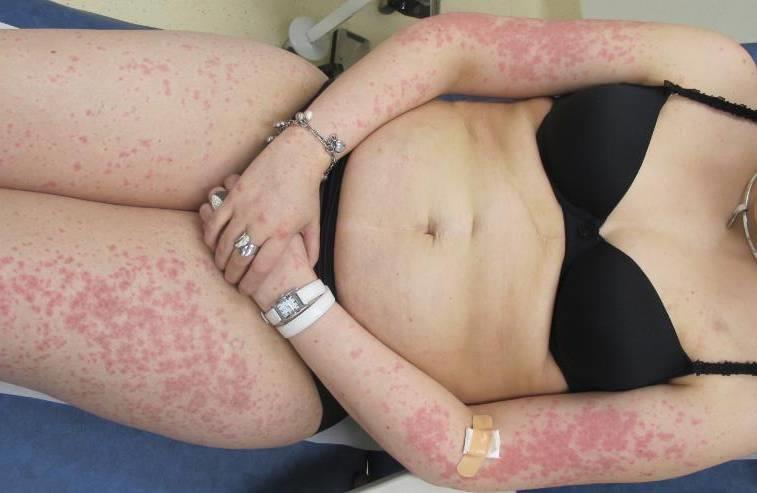
Grade 2 (moderate) rash
The corresponding NCI-CTCAE v5.0 definition for ‘grade 2 Rash maculopapular’ reads: macules/papules covering 10 - 30% BSA with or without symptoms (e.g., pruritus, burning, tightness); limiting instrumental activities of daily living (ADL); rash covering >30% BSA with or without mild symptoms.
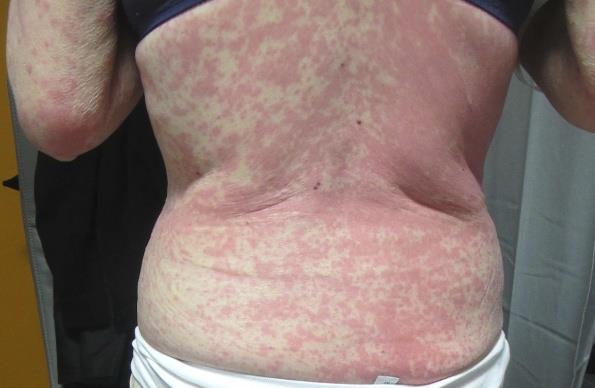
Grade 3 (Severe) rash
The NCI-CTCAE v5.0 definition for ‘grade 3 Rash maculopapular reads: Macules/papules covering >30% BSA with moderate or severe symptoms; limiting self-care ADL, which in majority of the cases is associated with local superinfection with oral antibiotics indicated.
Images courtesy of Siegfried Segaert, MD, PhD
Related Links
References
- National Cancer Institute Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events and Common Toxicity Criteria [v5.0]. 27 November 2017. (Accessed 15 April 2019).
- European Medicines Agency. Stivarga (regorafenib) Summary of Product Characteristics 2018.
- European Medicines Agency. Nexavar (sorafenib) Summary of Product Characteristics 2018.
- European Medicines Agency. Caprelsa (vandetinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Sutent (sunitinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Glivec (imatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Cabometyx (cabozantinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Cometriq (cabozantinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Kisplyx (lenvatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Lenvima (lenvatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Sprycel (dasatinib) Summary of Product Characteristics 2019.
- European Medicines Agency. Votrient (pazopanib) Summary of Product Characteristics 2018.
- European Medicines Agency. Rydapt (midostaurin) Summary of Product Characteristics 2018.
- De Wit M, et al. Support Care Cancer. 2014;22:837–846.
- Walko CM, Grande C. Semin Oncol. 2014;41:S17–S28.
- Autier J, et al. Arch Dermatol. 2008;144:886–892.
- Huang X, et al. Drug Des Devel Ther. 2008;2:215–219.