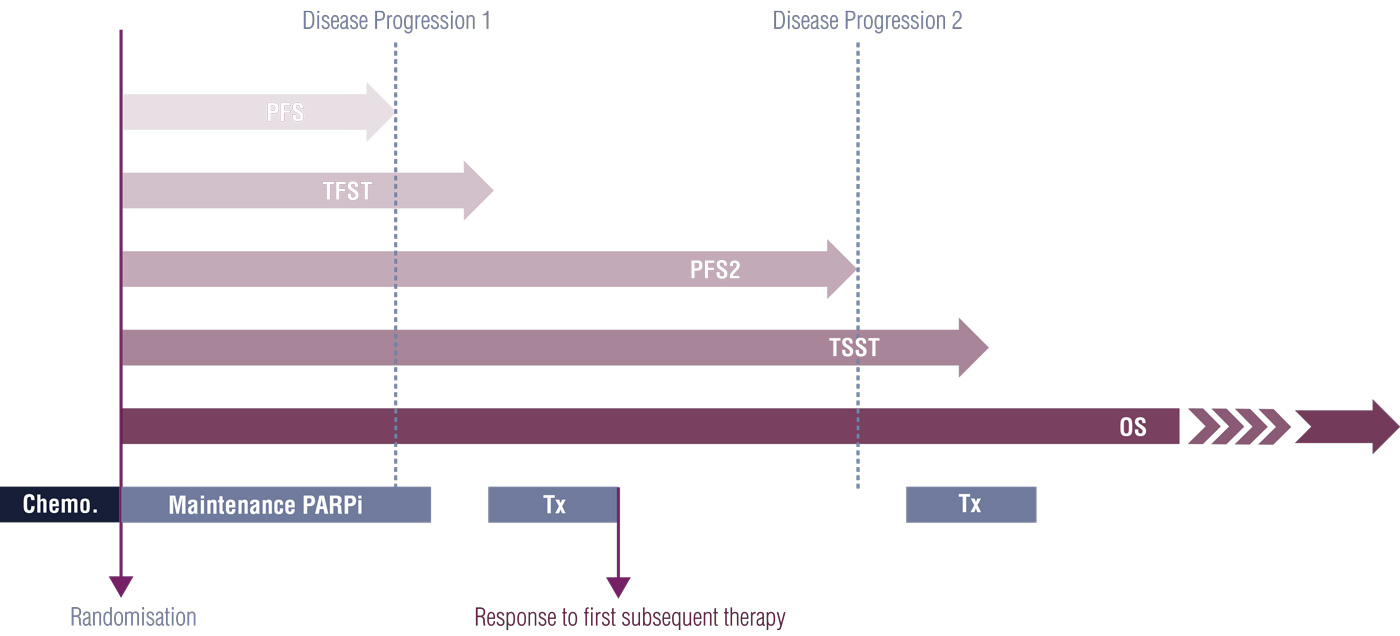
Intermediate Endpoints
For many indications, overall survival can be affected by additional treatments after progression, and the timeframe for analysis of overall survival may also be long [4]. This results in it being difficult to demonstrate overall survival benefit. To address these issues, novel clinical endpoints known as intermediate endpoints have been developed.
Intermediate endpoints occur after initial progression-free survival and before death (see Figure below) [4]. Intermediate endpoints complement progression-free survival where patients might experience prolonged progression-free survival or post-progression survival benefit in multiple subsequent lines of therapies [4].
Several intermediate endpoints have been used, mainly in ovarian cancer studies of PARP inhibitors, including Time to First Subsequent Therapy or death (TFST), Progression Free Survival 2 (PFS2), and Time to Second Subsequent Therapy or death (TSST). PFS2 is a useful endpoint to highlight whether the natural history of the disease is worsened by the study drug or not, and to demonstrate that there can be benefit beyond initial disease progression[4].
Figure 3: Implementation of intermediate clinical endpoints in PARP inhibitor trials and their positioning in the treatment course.
References
- Heeke AL, Pishvaian MJ, Lynce F et al. Prevalence of Homologous Recombination-Related Gene Mutations Across Multiple Cancer Types. JCO Precis Oncol 2018; 2018.
- Knijnenburg TA, Wang L, Zimmermann MT et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across The Cancer Genome Atlas. Cell Rep 2018; 23: 239-254 e236.
- Aguirre AJ, Nowak JA, Camarda ND et al. Real-time Genomic Characterization of Advanced Pancreatic Cancer to Enable Precision Medicine. Cancer Discov 2018; 8: 1096-1111.
- Matulonis UA, Oza AM, Ho TW, Ledermann JA. Intermediate clinical endpoints: a bridge between progression-free survival and overall survival in ovarian cancer trials. Cancer 2015; 121: 1737-1746.