Technical Procedures
As opposed to chemotherapies (ChTs) and tumour-targeted therapies, which aim to directly destroy cancer cells, immune checkpoint-directed therapies bind lymphocyte ligands or receptors to enhance the lymphocyte activation and allow a cytotoxic anti-tumour immune response. The first immune checkpoint-targeted therapies developed in the clinic were humanised or fully human monoclonal antibodies selected for their antagonistic properties against immune checkpoints such as CTLA-4, PD-1 and PD-L1. They have demonstrated promising clinical activity in more than 30 different cancer types in early phase trials. Patients with a tumour response share a common feature: their response is more durable than has been observed to date with ChTs and tumour-targeted therapies. This durability of tumour response has translated into significant overall survival (OS) benefits in several phase III clinical trials. Another characteristic of these drugs is their safety profile: they can trigger autoimmune and inflammatory toxicities in patients, so-called immune-related adverse events (irAEs).
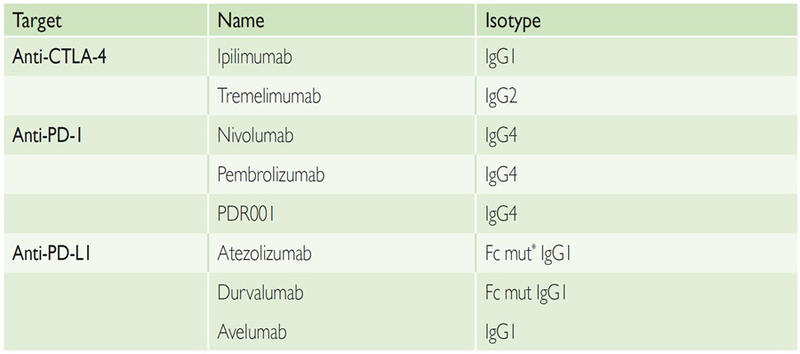
Different isotypes have been used so far in the clinic (Table 1). These antibodies usually have a long half-life and are usually infused intravenously (i.v.) with varying intervals of administration. Anti-PD-1 and anti-PD-L1 antibodies were initially developed on weight-based dosing. However, results from several clinical trials have shown no correlation between dose, efficacy and toxicity for anti-PD-(L)1, and most compounds are now developed with a flat dose, sufficient to saturate the target. For the anti-CTLA-4 antibody ipilimumab, there was no dose-limiting toxicity (DLT) identified in early phase trials. However, a recent randomised study in patients with metastatic melanoma (MM) has shown that ipilimumab was more active and more toxic at 10 mg/kg than the approved dose of 3 mg/kg. This dose–efficacy relationship of ipilimumab currently raises questions about the mechanism of action of anti-CTLA-4 antibodies and the optimal dose to be used when combined with anti-PD-(L)1 antibodies.
Table 1: First Generation of Immune Checkpoint-targeted Monoclonal Antibodies
New antibodies targeting inhibitory immune checkpoints such as LAG-3, T cell immunoreceptor with immunoglobulin and inhibitory motif (TIGIT), VISTA and TIM-3, and co-stimulatory checkpoints such as OX40, CD40, 4-1BB, GITR and ICOS are currently being evaluated.