IDH1 and IDH2 Mutations (IDH Mutations) in Cancer
The isocitrate dehydrogenase (IDH) family of enzymes comprises three isoforms located in the cytoplasm and peroxysomes (IDH1), and mitochondria (IDH2 and IDH3). IDH enzymes are involved in a number of cellular processes including mitochondrial oxidative phosphorylation, glutamine metabolism, lipogenesis, glucose sensing, and regulation of cellular redox status 1,2,3.
IDH1 and IDH2 are highly similar proteins, both catalyzing the reversible oxidative decarboxylation of isocitrate to alpha-ketoglutarate (αKG) while reducing NADP+ to NADPH, while IDH3 catalyzes the NAD+-dependent conversion of isocitrate to αKG in the tricarboxylic acid cycle (TCA cycle).
The IDH1 and IDH2 genes are located on chromosome 2q33.3 and 15q26.1, respectively. Mutations in the IDH1 and IDH2 genes have been identified in subsets of patients across a number of solid and haematologic malignancies, including glioma, acute myeloid leukaemia, myelodysplastic sybdrome, cholangiosarcoma and chondrosarcoma. Almost all IDH mutations are heterozygous somatic point mutations that cluster at the active sites of the IDH1 and IDH2 enzymes, leading to the substitution of the aminoacids arginine 132 in IDH1 and arginine 172 or 140 in IDH2. IDH-mutant enzymes gain neomorphic enzymatic activity, converting NADPH and αKG to NADP+ and D-2-hydroxyglutarate (D-2HG), an onco-metabolite that accumulates to high levels in IDH-mutant cells 4. The main theory behind D-2HG tumourigenesis is its ability to competitively inhibit αKG dependent dioxygenases, which are involved in a wide range of cellular processes (e.g. hypoxia, angiogenesis, maturation of collagens of the extracellular matrix, and regulation of epigenetics) 5,6,7. Excess of D-2HG inhibits key enzymes involved in histone- and DNA-demethylation, resulting notably in histone and DNA hypermethylation, which affects gene expression, and proliferation of mutant cells. Methylation profiling studies of IDH-mutant tumours have shown that they typically display CpG island methylator phenotype (CIMP) characterized by high degree of DNA hypermethylation in CpG-rich domains 8,9. Beside epigenetic reshaping, the presence of IDH mutation affects several cellular pathway including cellular metabolism and response to hypoxic and oxidative stress 6,7.
IDH Mutations in Glioma
IDH mutation testing is a valuable diagnostic, prognostic and predictive biomarker for the management of patients with glial tumours. IDH1 and IDH2 mutations occur in a mutually exclusive manner in nearly 80% of grades II and III oligodendrogliomas and astrocytomas and secondary glioblastomas (i.e. glioblastomas that had progressed from lower grade gliomas) 10,11,12,3,13. Conversely, IDH mutations are found in only 6% of patients with primary glioblastoma. Molecular classification of gliomas divides these malignancies primarily into IDH-mutant and IDH-wild tumours, the latter being mainly represented by World Health Organization (WHO) grade IV primary glioblastomas 14. The presence of IDH mutations defines a distinct tumour subset with specific clinical and molecular characteristics, suggesting that these predominantly grades II and III tumours have distinct pathogenic origins from that of primary IDH-wild type glioblastomas 10,11,12,3. In gliomas, clinical features that have been associated with the presence of IDH mutation include younger age at presentation, tendency for frontal lobe location, more frequent non-enhancing tumour infiltrative component and more favourable outcome.
The most common IDH1 mutation – accounting for approximately 90% of all IDH mutations – causes an amino acid change from arginine to histidine (R132H) in the active site of the enzyme 13. Besides IDH1 R132H, rarer mutations affect either IDH1 at Arg132 (including R132S, R132C, R132G, and R132L substitutions), or IDH2 at Arg172 (R172K) 13.
Molecular studies of IDH-mutant gliomas have established that the IDH mutation is an early causative event in the pathogenesis of this brain tumour subset 12,14. Accordingly, the IDH mutation is often referred as a “trunk” (or initiating) event in the clonal evolutionary tree of IDH-mutant gliomas. Other molecular alterations frequently found in IDH-mutant tumours include 1p/19q co-deletion, CIC, FUBP and TERT promoter mutations, which characterize oligodendroglial tumours, while TP53 and ATRX mutations are found in astrocytic tumours 11,12,13,14. IDH-mutant gliomas typically present with hypermethylator phenotype (CIMP) and promoter methylation of the methyl-guanine methyl transferase (MGMT) gene, which have been associated with more favourable response to alkylating agents. As disease progresses, tumour cells often acquire additional oncogenic alterations, notably involving cell cycle regulation and growth control pathways, overall resulting in more malignant behaviour 15.
IDH Mutations as a Diagnostic Biomarker in Glioma
IDH mutations are valuable diagnostic marker that helps the differential diagnosis of low-grade glioma from reactive gliosis and other IDH-wild type tumour entities 16. Indeed, the presence of the IDH mutation is a pathognomonic biomarker that indicates a glioma entity in the setting of brain tumours. Thereby the detection of even a single IDH1 R132H-positive cell strongly supports the diagnosis of a diffusely infiltrating glioma.
IDH mutations are seen mainly in WHO grades II and III gliomas and secondary glioblastoma. Therefore, the identification of an IDH mutation supports the differential diagnosis between an anaplastic glioma and a glioblastoma. The presence of an IDH mutation in glioblastoma is suggestive of secondary glioblastoma that has transformed from a low-grade precursor 17.
IDH Mutations as a Prognostic Biomarker in Glioma
The presence of IDH mutation is a strong prognostic biomarker in patients with glioma, associated with a favourable outcome independent of age and grade 18. Conversely, the absence of IDH mutation in grade II/III gliomas marks a distinct IDH-wild type subgroup characterized by poor prognosis 10. The prognostic strength of IDH mutation is emphasised by the fact that survival of patients with IDH-mutated glioblastoma is more favourable than for non-mutated grade III astrocytoma 19.
Studies conducted in both anaplastic gliomas and glioblastomas have demonstrated that the co-occurrence of both mutated-IDH and methylated MGMT promoter has stronger prognostic value than either one of these genetic aberrations alone, associated with improved survival irrespective of treatment 20,21,22.
IDH as a Predictive Biomarker in Glioma
IDH mutation has predictive value for response to chemotherapy in anaplastic gliomas. Extended follow-up of a large randomised controlled trial (RTOG 9402) that compared procarbazine/lomustine/vincristine (PCV) chemotherapy in combination with radiotherapy to radiotherapy alone demonstrated a survival advantage of the combination in IDH-mutant anaplastic gliomas 23. Median overall survival for patients with IDH-mutant tumours was 5.7 years for patients treated with radiotherapy alone versus 9.4 years for patients who received radiotherapy plus PCV. Conversely, in the same trial, the addition of PCV chemotherapy to radiotherapy did not prolong median survival in patients with IDH wild-type tumours, (1.3 v 1.8 years).
Benefit of first-line PCV in combination with radiotherapy to radiotherapy alone has also been demonstrated in patients with “high-risk” grade II IDH-mutant gliomas (i.e. patients younger than 40 years of age who had undergone subtotal resection or biopsy or patients who were 40 years of age or older). Among patients with IDH1 mutations, those that received early PCV plus radiotherapy had longer progression-free survival than those who received radiation therapy alone. However, in the latter trial, the number of events among patients without IDH mutation was too small to determine an association with treatment effect in this subgroup 24.
In addition, IDH mutation could serve as a potential therapeutic target as inhibitors of mutant IDH have entered clinical trials for patients with IDH mutations. AG-120 and AG-221 are first-in-class, oral, selective inhibitors of the IDH1 and IDH2 mutant enzymes, respectively. Preclinical studies have shown that they inhibit mutant IDH activity and D-2-hydroxyglutarate accumulation, and phase I studies evaluating for both compounds are ongoing in patients with IDH-mutant gliomas (NCT02073994 and NCT02273739). Vaccination against IDH1-mutant specific peptides represent an alternative approach that is currently being investigated in WHO grade II-IV IDH1-mutant gliomas in phase I trials (NCT02193347, NCT02454634). The clinical benefit of mutant IDH inhibitors/vaccines remains unsettled, but if introduced into clinical practice, mutated-IDH may act as a predictive biomarker for response to these therapies.
IDH Testing in Glioma
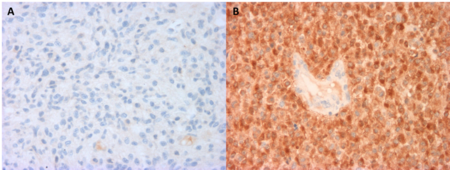
According to the 2016 WHO central nervous system tumour classification, assessment of IDH mutation status is recommended for all patients with grades II and III gliomas, and in patients younger than 55 years with glioblastoma 25. The most frequent IDH1 mutation can be detected by immunohistochemistry using the anti-IDH antibody that recognises the R132H mutated protein 26. Less frequent IDH1 and IDH2 mutations can only be identified by DNA sequencing.
IDH1 testing by immunohistochemistry is a reliable diagnostic method with high sensitivity/specificity, combined with other advantages as compared to DNA sequencing (i.e., time and cost-effectiveness, ability to detect few single positive cells which can be missed by sequencing tests). IDH1 immunohistochemistry can be performed on fixed and paraffin embedded tissue samples. The sensitivity and specificity of immunohistochemistry are reported to be higher than that of sequencing 26, but the latter technique is useful for identifying the rarer mutations not identified by immunohistochemistry. If immunohistochemistry is negative or inconclusive in lower grade tumours or suspected secondary glioblastoma, direct sequencing of the IDH1 and IDH2 genes by PCR- or next generation sequencing (NGS)-based methods should be carried out to screen for non-IDH1-R132H mutations 25. The choice of technique depends on laboratory expertise, equipment available for testing, and the clinician and pathologist’s preference.
Non-invasive methods to detect IDH mutation are under development, including detection of IDH1 mutation in the plasma by digital PCR 27 and magnetic resonance spectroscopy that allows detection of abnormal accumulation of 2-hydroxyglutarate within the tumour, which have the potential to assist monitoring of treatment response 28.
Interpretation of Immunohistochemistry Results
Aside from a few exceptions, for the majority of gliomas with IDH1-R132H mutation, cytoplasmic immunostaining is detected for all identifiable tumour cells within a sample 26. A positive immunoreaction is generally reliable at differentiating tumour tissue, but a negative reaction does not rule out mutation. The R132H antibody detects approximately 90% of IDH mutation. To avoid false-negative misinterpretation of results as IDH wild-type, immunonegative low-grade should be considered for DNA-based IDH testing for one of the rare IDH1 or IDH2 mutations 25.
Ensuring Quality of IDH Testing
It is important that immunostaining protocols and reactions and interpretation of results are standardised. IDH1-R132H immunostaining can be performed using standard inventory routinely used for diagnostic histopathological workup. Detailed guidance on both manual and automated immunostaining protocols and interpretation of results has been published 29. Anti-IDH1-R132H immunostaining protocols should be optimised with the inclusion of positive and negative controls 29. The immunostaining protocol should be optimised to show distinct tumour cell labelling in IDH-mutant cases (e.g., oligodendrogliomas) with little or no background staining, and IDH-wild type cases (e.g., primary glioblastomas) should be unambiguously negative 29.
Patient Selection
Given their diagnostic, prognostic and potential predictive value, assessment of IDH1/2 status should be integrated into routine clinical practice for all patients with primary brain tumours 17,29. On the basis of the RTOG 9402 trial findings, patients with IDH-mutant anaplastic glial tumours should not be treated with radiotherapy alone, but instead receive early alkylating chemotherapy with radiotherapy 30. When a medical treatment is needed, a similar approach should be considered in a subset of patients with grade II IDH-mutant gliomas.
References
- Cairns R, Mak T. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discovery 2013; 3: 730-41.
- Dang L, Yen K, Attar EC. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol 2016; 27: 599 – 608.
- Mondesir J, Willekens C, Touat M, et al. IDH1 and IDH2 mutations as novel therapeutic targets: current perspectives. Journal of Blood Medicine 2016; 7: 171-80.
- Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009; 462(7274):739-44.
- Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenase. Cancer Cell 2011; 19(1):17-30.
- Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science 2009; 324(5924):261-5.
- Grassian AR, Parker SJ, Davidson SM, et al. IDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolism. Cancer Res 2014; 74(12):3317-31.
- Figueroa M, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell 2010; 18: 553-67.
- Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is suffi cient to establish the glioma hypermethylator phenotype. Nature 2012; 483: 479–83.
- Parsons D, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008; 321: 1807–12.
- Yan H, Parsons D, Jin G, et al.IDH1 and IDH2 Mutations in Gliomas. N Engl J Med 2009; 360: 765-773.
- Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol 2009; 174: 1149-53.
- Hartmann C1, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 2009; 118: 469-74.
- Cancer Genome Atlas Research Network, Brat D, Verhaak R, Aldape K, et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 2015; 372: 2481–98.
- Bai H, Harmanci AS, Erson-Omay EZ, et al. Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat Genet 2016; 48(1):59-66.
- Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 2014; 15: 395-403.
- Stupp R, Brada M, van den Bent M, et al. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 (Suppl 3): iii93-101.
- Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol 2009; 27: 4150–54.
- Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 2010; 120(6):707-18.
- van den Bent M, Dubbink H, Sanson M, et al. MGMT promoter methylation is prognostic but not predictive for outcome to adjuvant PCV chemotherapy in anaplastic oligodendroglial tumors: a report from EORTC Brain Tumor Group Study 26951. J Clin Oncol 2009; 27: 5881-6.
- Wick W, Meisner C, Hentschel B, et al. Prognostic or predictive value of MGMT promoter methylation in gliomas depends on IDH1 mutation. Neurology 2013; 81:1515–22.
- Molenaar R, Verbaan D, Lamba S, et al. The combination of IDH1 mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol 2014; 16: 1263–73
- Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol 2014; 32(8):783-90.
- Buckner JC, Shaw EG, Pugh SL, et al. Radiation plus Procarbazine, CCNU, and Vincristine in Low-Grade Glioma. N Engl J Med 2016; 374:1344-1355.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016; 131(6):803-20.
- Capper D, Weissert S, Balss J, et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol 2010; 20 : 245–54.
- Boisselier B, Gallego Perez-Larraya J, Rossetto M, et al. Detection of IDH1 mutation in the plasma of patients with glioma. Neurology 2012; 79(16):1693-8.
- Choi C, Ganji S, DeBerardinis R, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med 2012; 18: 624-29.
- Preusser M, Capper D, Hartmann C. IDH testing in diagnostic neuropathology: review and practical guideline article invited by the Euro-CNS research committee. Clin Neuropath 2011; 30: 217-30.
- Dunn G, Andronesi O, Cahill D. From genomics to the clinic: biological and translational insights of mutant IDH1/2 in glioma. Neurosurg Focus 2013; 34: E2.